|
China Shineway Pharmaceutical Group Limited
(Stock Code: 2877)
|
Listing Date: |
2 December 2004 |
|
Offer Price: |
HK$ per share |
|
Par Value: |
HK$0.10 |
|
No. of Shares Offer : |
200,000,000 Shares |
|
No. of Placing Shares: |
180,000,000 Shares |
|
No. of Public Offer Shares |
20,00,000 Shares |
|
Market Capitalization: |
HK$ 2960-3488 million |
|
Sponsor: |
Cazenove Asia Limited |
|
Chairman: |
Mr. Li Zhenjiang |
|
Fund Raising |
HK$740-872 million |
Substantial Shareholder:
Mr. Li Zhenjiang 's Family: 75% interest
Company Subsidiaries:
- Yuan Da International Limited 100% Investment holding
- Hong Zhan International Limited 100% Investment holding
- Shineway Pharmaceutical Sales Co. Limited 80% Trading of Chinese Pharmaceutical products
- Shineway Pharmaceutical Company Limited 100% Research and development, manufacture and trading of Chinese pharmaceutical products
- Hebei Shineway Pharmaceutical Company Limited 100% manufacture and trading of Chinese pharmaceutical products
- China Shineway Pharmaceutical (Hong Kong) Limited 100% Trading of Chinese Pharmaceutical products
COMPANY OVERVIEW
The Group is principally engaged in the development, manufacture and sale of modern Chinese medicines in the PRC and also produces and sells series of western pharmaceuticals. Base on the China Medical Statistical Yearbook 2002, the Group was the largest manufacturer of three of its principal products, Xin Nao Qing soft capsules, Qing Lai Ling injection and Shen Mai injection, in the PRC 2002. The Group sells its products under its principally brand "SHINEWAY".
China has been one of the major countries in terms of expenditure on health. According to he China Medical Statistical Yearbook, consumption of Chinese medicines has been getting more popular in China recent years. Sales of Chinese medicines grew ar a CAGR of approximately 21.4% between 1999 and 2003, compared to the CAGR of sales of western medicines of approximately 16.1%.
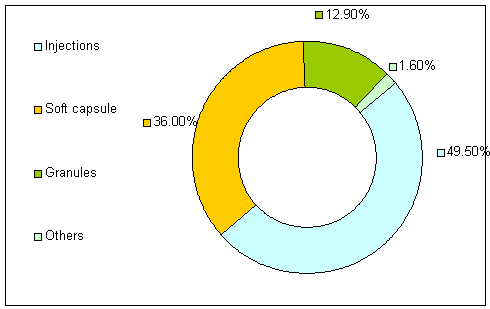
The Group had a range of 31 prescription and 14 OTC medicines as at 30 June 2004, since when 7 of such prescription medicines have obtained OTC registration for sale in retail pharmacies. In addition, the Group has obtained production approvals to produce, among others, 180 other pharmaceuticals. The Group's 45 medicines regularly offered are primarily focuses on (I) the treatment of disease commonly affecting middle and old aged people and/ or children, including cardiovascular diseases, respiratory system disease, colds and fever and digestive system diseases; or (II) anti-viral treatment. 21 of the Group's regular products are included in the Insurance Catalogue. The group sells its products mainly in the forms of injection, soft capsules and granules, each ready for immediate therapeutic use.
The group accomplished a turnover of approximately RMB $ 430,887,000, RMB$477,930,000 and RMB$604,042,000 respectively for the three years ended 31 December 2003.
COMPETITIVE ADVANTAGES
The Directors believe the Group has the following principal strengths:
- Production know-how combined with economies of scale
- Strong brand reputation built on high quality products and customers services;
- Established track record in research, development and commercialization of products and development of improved production techniques
- An extensive and structure distribution network covering 30 provinces and municipal cities in the PRC.
- A diversified portfolio of products targeted at key segments of the pharmaceuticals market in the PRC.
- An experienced and committed management team with a stringent management system and incentivised staff.
RISK FACTORS
A large proportion of the Groups revenue has been derived from certain major products during the track record period
The Group relies on distributors for the sales of its products
The Group relies on research institutions, universities and hospitals in the PRC for research and development of new products
There is no assurance that the Group's future research and development projects will be successful
The Group has only obtained protection for certain of its products and other pharmaceutical manufacturer in the PRC are entitled to produce many of the products produced by the Group
Rights associated with the SHINEWAY brand name and other registered trademarks owned the Group may be infringed or the reputation associated with such brand name may suffer result of use by Shineway Medical and its subsidiaries and there is no assurance that the Group's efforts to promote its brand in the future will be successful
There can be no assurance that the product liability insurance carried by the Group will sufficient to cover any liability of the Group
The Group relies on certain key personnel
There is no assurance that the Group will be able to successfully implement its business plan.
There is no assurance that the Group will be able to maintain or increase historical levels revenue or profitability
There is no assurance that the members of the Group will continue to benefit from preferential treatment
The Group relies on the operation of its production facilities
There is no assurance that the Company will declare dividend in the future
BREAKDOWN OF INCOME SIX MONTHS ENDED 30 June 2004
FINANCIAL RECORD
|
Year ended
31 Dec 2001
(RMB'000) |
Year ended
31 Dec 2002
(RMB'000) |
Year ended
31 Dec 2003
(RMB'000) |
Six months ended
30 June 2004 (RMB'000) |
|
Turnover |
430,887 |
477,930 |
640,042 |
336,546 |
|
Profit before tax |
108,975 |
126,344 |
185,082 |
161,151 |
|
Net profit |
71,676 |
77,412 |
151,370 |
146,061 |
|
Total Assets |
581,225 |
756,053 |
631,062 |
377,648 |
|
Total Liabilities |
338,519 |
453,650 |
235,326 |
234,178 |
|
Total equities |
242,706 |
302,403 |
395,736 |
143,470 |
PROFIT FORECAST
Forecast for the year ending December 31, 2004
|
Forecast profit after taxation and minority interests but before extraordinary items |
Not less than RMB 250 million
|
|
Forecast earnings per Share (Weighted average) |
RMB0.41 (HK$0.39) |
|
Forecast earnings per Share (Pro forma fully diluted) |
RMB0.31 (HK$0.29) |
FUTURE PLANS
The Group's objective is to become the leading supplier of modern Chinese medicine products in the PRC. The Directors believe that, with the implementation of GMP and GSP standards in the PRC on June 30 and by 31 December 2004 respectively, pharmaceutical manufacturers that are unable to comply with GMP standards and pharmaceutical distribution enterprises that unable to comply with GSP standards will be phrased out. The Directors believe that the pharmaceutical market in the PRC will eventually be dominated by a few market leaders with nationwide brands, larger scale production, technologically advanced products and an extensive and marketing networks.
To archive its objective, the Group has implemented the following strategies:
- Market development and network expansion
- Production capacity expansion
- Further strengthening of research and development capability
- Enhancement of information management and logistics system
- Pro-active development of supplier relationships
USE OF PROCEEDS
The net proceeds of the International Offering, after deducting the underwriting fees and related listing expenses payable by the Company (assuming the Over-allotment Option is not exercised and assuming an Offer Price of HK$3.70 per Share, being the lowest end of the proposed offer price range of HK$3.70 to HK$4.36 per Share) are estimated to be approximately HK$690 million, The Group at present intends to apply the net proceeds as follows:
|
Expanding sales and marketing network and developing markets |
35.36 |
|
Expanding and upgrading production capabilities |
30.43 |
|
Strengthening research and development capacities |
11.88 |
|
Upgrading information management and logistics systems |
10.29 |
|
Enhancing relationship wit suppliers and production bases |
2.32 |
|
As general working capital |
9.72 |
|
 Stock Information Provided by Infocast Limited
[
Disclaimer
]
Stock Information Provided by Infocast Limited
[
Disclaimer
]
